- How Many Electrons Protons And Neutrons Are In Fluorine 23
- Fluorine Protons Neutrons And Electrons
- Fluorine Protons Electrons
Fluorine Coupling to 1H Coupling between hydrogen and fluorine (spin 1/2) is very strong. Typical 2J coupling constants are about 48 Hz. Longer range coupling is smaller. Typical 4J coupling constants are about 4 Hz. The figure below contains the NMR spectrum for fluoroacetone. The nuclear spin of fluorine is 1/2. This means that the proton. The symbol for fluorine is F. In an atom, protons and neutrons reside in the nucleus, and electrons revolve around it. To maintain the overall neutrality of the atom, the number of protons equals. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
A team of U.S. physicists has created a new isotope of fluorine, fluorine-13 (13F), via a charge-exchange reaction between a beam of oxygen-13 (13O) and a beryllium-9 (9Be) target.
Charity et al. discovered a new isotope of fluorine using the High Resolution Array at NSF’s National Superconducting Cyclotron Laboratory. Image credit: Michigan State University.
Fluorine is the chemical element with the symbol F and atomic number 9.
It is the most chemically reactive element on the periodic table and the lightest member of the halogen elements.
At standard conditions fluorine exists as a highly toxic, pale yellow diatomic gas.

It has 18 known isotopes ranging from 13F to 31F (with the exception of 30F) and two isomers (18mF and 26mF).
Only one isotope of fluorine occurs naturally, the stable isotope 19F.
“Study of exotic nuclei with such large excesses of neutrons or protons is of considerable interest in understanding the synthesis of elements, even though their lifetimes are extremely short,” said Professor Robert Charity, a researcher in the Departments of Chemistry and Physics at Washington University, St. Louis.
“Many of these isotopes have exotic properties,” he noted.
The new isotope of fluorine, 13F, is four neutrons removed from the proton drip line, the boundary that delimits the zone beyond which atomic nuclei decay by the emission of a proton.
“All the new isotopes are very proton-rich and unstable to the emission of protons,” Professor Charity said.
“The highest-energy protons inside these isotopes can tunnel through the Coulomb barrier and escape.”
“The initial purpose of the experiment was to make a new isotope of oxygen, dubbed featherweight oxygen,” he added.
“After making that discovery, we went through our data again with great care and teased out evidence for 13F.”
The 13F isotope was observed following a charge-exchange reaction with a beam of 13O (a neutron in 13O is removed and replaced by a proton) and a 9Be target.
“Such charge-exchange reactions have not typically been used for the creation of the very proton-rich isotopes in the past,” Professor Charity said.
“However, we are already planning a search for another new isotope using this reaction mechanism.”
The team’s results were published in the April 2, 2021 issue of the journal Physical Review Letters.
How Many Electrons Protons And Neutrons Are In Fluorine 23
_____
R. J. Charity et al. 2021. Observation of the Exotic Isotope 13F Located Four Neutrons beyond the Proton Drip Line. Phys. Rev. Lett 126 (13): 132501; doi: 10.1103/PhysRevLett.126.132501
Fluorine is a remarkable element with very special chemical and nuclear properties that give it a unique role in realizing the goal of generating energy from thorium.
Pure fluorine is so reactive with other materials that it is never found in that form in nature—it is always chemically bound to other materials.
Each of these naturally occuring minerals contain fluorine bound to other elements. The pink crystal is fluorite, which consists of a single calcium atom bound to two fluorine atoms. The milky white mineral is cryolite, which is three sodium atoms and an aluminum atom together with six fluorine atoms (Na3AlF6). Fluorapatite is another form of fluorine-containing material, formed from calcium, phosphate ions, and fluorine. A layer of fluorapatite forms on teeth in the presence of dissolved fluoride, protecting them against decay.
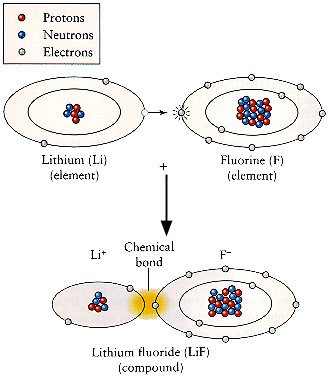
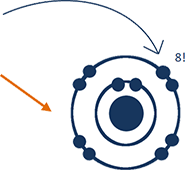
The reason that fluorine is never found by itself, and why it always wants to combine with other elements, is the configuration of its electrons in their orbits. Atoms have certain orbits for their electrons, and each orbit holds certain numbers of electrons. When an orbit is full of electrons, then the atom is very “happy” and doesn’t want to chemically react with something else. Fluorine only needs one more electron to fill its last orbit. It wants that electron very, very badly. But if it can get it, it becomes very “happy” and doesn’t want to react with anything else.
So there is a great principle here—if we can combine fluorine with another material that very much wants to get rid of an electron, then both materials can be very “happy” and will no longer be chemically reactive with other things. For fluorine, the logical materials with which to combine are those that have one or two electrons they’d like to get rid of, such as lithium, sodium, potassium, beryllium, magnesium, calcium. All of these “combining” elements are found on the left-hand side of the periodic table.
For instance, lithium and fluorine combine in a way that makes both of them very “happy” and chemically unreactive. Lithium has one too many electrons for a full orbit, and fluorine has one too few. Put them together, and fluorine will take the electron from lithium and enjoy its full orbit of electrons. Lithium will have an empty orbit of electrons. But now fluorine has ten electrons but only nine protons. Its charge is unbalanced. It has an extra electron, and so it has a negative charge. An atom with one or more extra charges is called an “ion”. A negatively charged ion like fluorine is called an “anion”. Lithium on the other hand has three protons but only two electrons. It is also electrically unbalanced, but with a positive charge. Positively-charged ions are called “cations”. So long as anions and cations are equal in number to one another, then the overall charge is balanced and the material is very unreactive.
This is the basic principle of a salt. A “salt-making” element like fluorine, chlorine, or iodine, combines with a metal like lithium or magnesium and they form a cation-anion pair that is very chemically stable.
Another stable fluoride salt is sodium fluoride, and the odds are that you brushed your teeth this morning with some sodium fluoride salt mixed in your toothpaste.
When fluorine has formed an ion, we typically don’t call it “fluorine” anymore. Instead we call it a “fluoride ion”. Fluoride means that the fluorine is in its ionic, unreactive state, not in its elemental, reactive state. That’s also why we call salts like lithium fluoride by that name instead of “lithium fluorine”. So when you hear the word “fluoride” you know that you are talking about a fluorine in the form of a salt.
Because metals and fluorides form stable compounds, fluorine was used to transform metallurgy by allowing certain metals to be extracted that could not be purified in any other way. The most important example is aluminum. Before fluoride technology, aluminum was more valuable than gold, despite the fact that it is one of the most common elements on Earth. Aluminum was always found naturally bound to oxygen, and the bond was so strong that typical metallurgical techniques could not break the bond between the aluminum and the oxygen.
Fluorine could, however. Aluminum fluoride was even more chemically stable than aluminum oxide, so aluminum would be formed into a salt, melted, and then electricity would be driven through the salt to separate metallic aluminum from fluorine. The fluorine is recycled to continue the process and aluminum became incredibly inexpensive. Now we make soda cans from aluminum, but more importantly we make airplanes from aluminum, and flight would not be possible without the strength of lightweigh aluminum. So fluoride technology has been a very important contributor to making air travel possible.
Fluorine also has notable nuclear properties. Natural fluorine has only one isotope, fluorine-19, with nine protons and ten neutrons. Often, elements have several stable isotopes. But fluorine only has one. Even more importantly, that single isotope of fluorine has a very low neutron absorption cross section. If fluorine-19 was even slightly more neutron-absorptive, molten-salt reactors would not be possible.
During the Manhattan Project, the first attempts were made to separate the two isotopes of uranium, and the remarkable properties of fluorine came to the rescue. Six fluorine ions would bond with uranium to form uranium hexafluoride, which was solid at room temperature but would sublimate to form a gas if heated to a modest temperature. The mass of uranium hexafluoride was slightly different if the uranium had 146 or 143 neutrons, corresponding to whether it was uranium-238 or uranium-235. The isotope separation techniques used for uranium depends on this slight mass difference. If fluorine had had several isotopes with different atomic masses, then uranium hexafluoride would have numerous different molecular masses. But since fluorine had only a single atomic mass, the molecular mass of uranium hexafluoride had only two values: 352 or 349 (238 + 6*19 or 235 + 6*19).
Uranium enrichment was made possible by the remarkable chemical and nuclear properties of fluorine. The almost magical combination of uranium and fluorine was a key ingredient in nearly all of the nuclear activities that would follow, including, ironically the realization of energy from thorium.
Fluorine Protons Neutrons And Electrons
Fluorine was “discovered”, or more accurately, fluorine was isolated from all other elements for the first time by a French chemist named Henri Moissan. Scientists had believed that an element such as fluorine must exist but it had been very challenging to isolate it from all other elements due to the extreme chemical reactivity of fluorine when it is isolated from the stabilizing effects of other materials. Some scientists had even died trying to isolate fluorine.
Moissan succeeded by using electricity to break apart a solution of potassium hydrogen difluoride (KHF2) in liquid hydrogen fluoride (HF). In this technique, the electricity caused the HF to break apart and pure hydrogen gas to form at the anode of the device and pure fluorine gas at the cathode of the device. This is essentially the same way we produce pure fluorine gas today, and a technique such as this will probably be used inside the liquid-fluoride thorium reactor to reproduce hydrogen and fluorine gases as reactants from HF.
Fluorine Protons Electrons
Moissan won the 1906 Nobel prize in chemistry for his isolation of fluorine in 1886 and he went on to study many other uses for fluorine.





