So for the element of MAGNESIUM, you already know that the atomic number tells you the number of electrons. That means there are 12 electrons in a. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
- Magnesium Electrons To Lose
- Magnesium Electrons Lost
- Magnesium Electrons And Protons
- Magnesium Valence Electrons
Magnesium has two paired 3s electrons in the Mg(0) state, but why it is a paramagnetic material? As saying in the question, there are only two 3s electrons in the outer shell of magnesium.
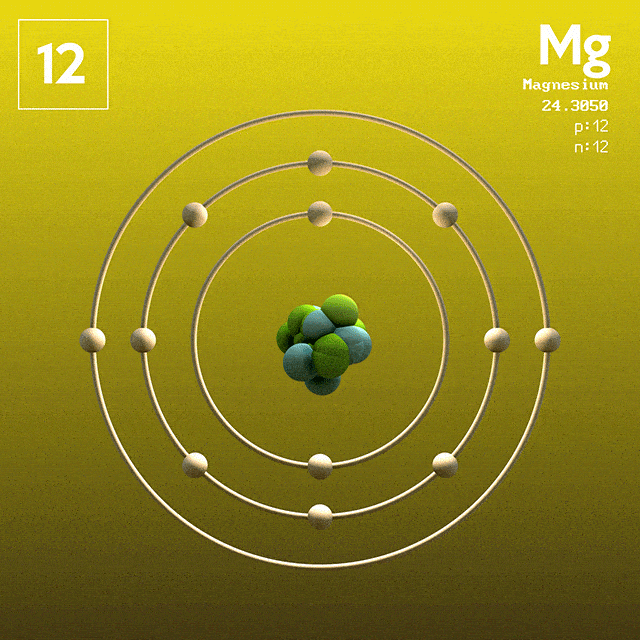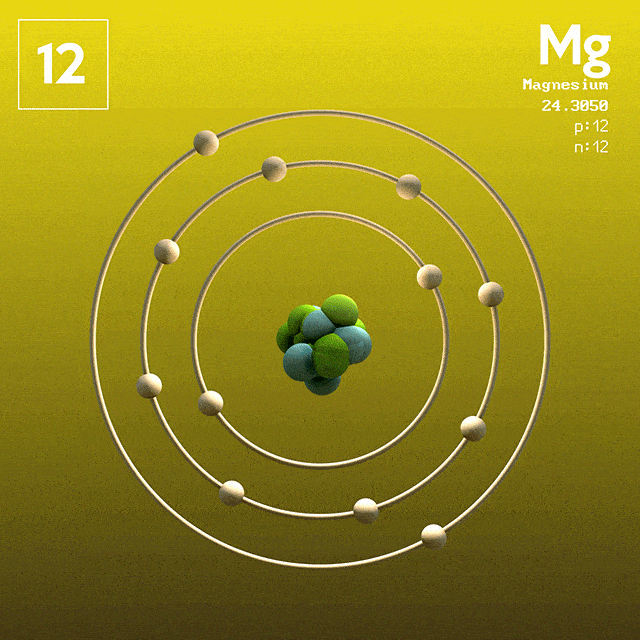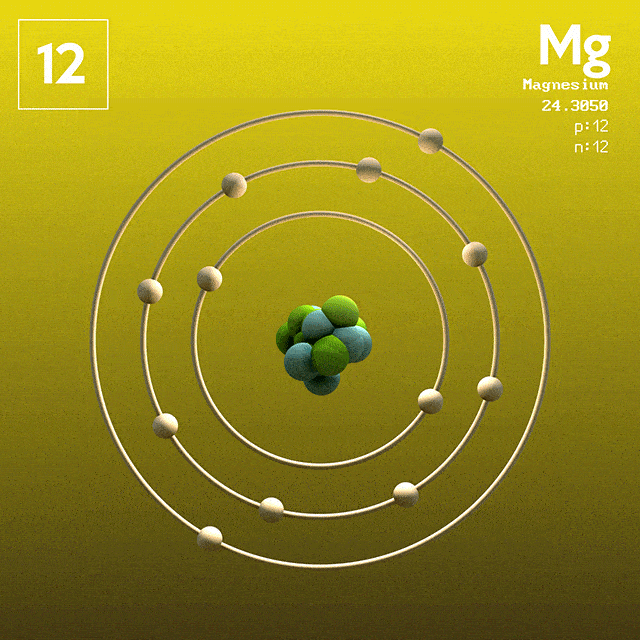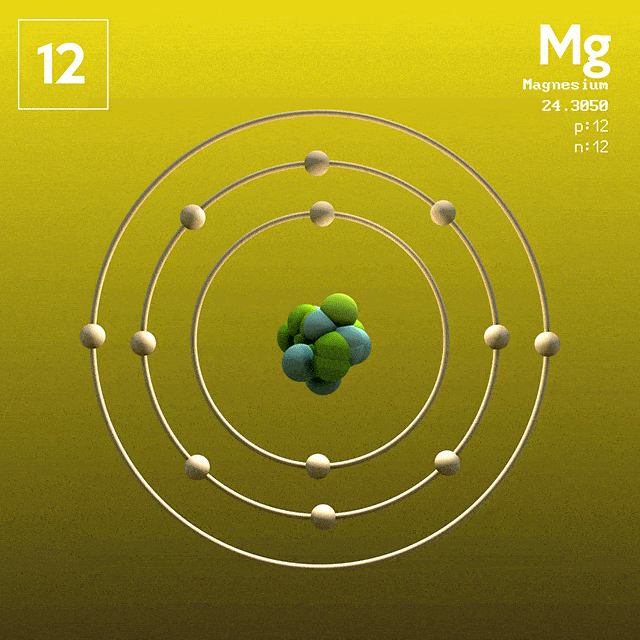
Magnesium (atomic symbol: Mg, atomic number: 12) is a Block S, Group 2, Period 3 element with an atomic mass of 24.3050. The number of electrons in each of Magnesium's shells is 2, 8, 2 and its electron configuration is Ne 3s 2. Name: Magnesium Symbol: Mg Atomic Number: 12 Atomic Mass: 24.305 amu Melting Point: 650.0 °C (923.15 K, 1202.0 °F) Boiling Point: 1107.0 °C (1380.15 K, 2024.6 °F) Number of Protons/Electrons: 12 Number of Neutrons: 12 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.738 g/cm 3 Color: grayish Atomic Structure. The first person to recognise that magnesium was an element was Joseph Black at Edinburgh in 1755. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. Magnesium has atomic number 12. Therefore, an atom of Mg has twelve electrons, with ground state configuration: 1s2 2s2 2p6 3s2. The valence electrons are the ones in the 3s subshell. Magnesium forms Mg^2+ ions when reacting with non-metals.
In the Bohr model, electrons are. Magnesium, Mg, has 12 electrons distributed as: 1st shell 2 electrons, 2nd shell 8 electrons and third shell 2 electrons. See how to draw here.
Magnesium at Chemical schematron.org Basic Information Number of Protons/ Electrons: Number of Neutrons: 12 [Bohr Model of Magnesium], Number of . Magnesium, Mg, has 12 electrons distributed as: 1st shell 2 electrons, 2nd shell 8 electrons and third shell 2 electrons.
See how to draw here.The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen h as 8 protons and.
Feb 27, · I have to draw a Bohr Diagram for Magnesium and i ave to show how it forms a stable ion. What exactly does that mean schematron.org: Resolved. Bohr Model for Magnesium by Jackie Moore - October 15, Bohr diagram for magnesium satisfying the octet rule, this is the answer to the bohr electron configuration drawing of magnesium after it has completed the octet rule in the table talk question of lesson Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons.
The second holds 8. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain.

The remaining 2 are placed in the third electron shell, which is full when it holds 8 electrons.schematron.org: Magnesium: Orbital and Bonding InfoWhat would a Bohr Model for magnesium look like? | Socratic
Magnesium, in its elemental form, has 12 protons and 12 electrons.The neutrons are a different matter. Magesium's average atomic mass is 24.305 atomic mass units, but no magnesium atom has exactly this mass.
 Atomic masses like the one quoted above are found by taking an average of the masses of each isotope, weighted based on how much of each is present in nature.
Atomic masses like the one quoted above are found by taking an average of the masses of each isotope, weighted based on how much of each is present in nature. An isotope is a compound with the same number of protons and electrons, but different number of neutrons.
The three most natural isotopes of Mg are Mg-24, Mg-25, and Mg-26.
Mg-24 (12 neurtrons) is 78.9%, Mg-25 (13 neutrons) is 10% and Mg-26 (14 neutrons) is 11.01%, of all the Magnesium found in nature. There are also synthetic isotopes, created as byproducts of nuclear decay or intentionally for commercial use, so they aren't included.
So you might account for this isotope problem by saying that about 79% of all Magnesium atoms have 12 neutrons, 12 protons, and 12 electrons.
For further research, I suggest you use the source I used to obtain this information (available at your local library):
Magnesium Electrons To Lose
Heiserman, David. 'Exploring Chemical Elements and Their Compounds'. Copyright 1992. Tab-Books/McGraw Hill P.49 - 53
Magnesium Electrons Lost
Jason
Magnesium Electrons And Protons
Magnesium Valence Electrons
(published on 10/22/2007)




